MAKE A MEME
View Large Image
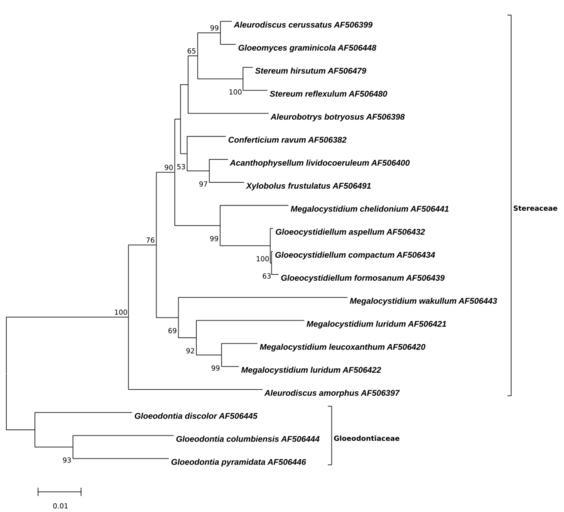
| View Original: | Megalocystidium-Minimum Evolution-Tree.svg (148x134) | |||
| Download: | Original | Medium | Small | Thumb |
| Courtesy of: | commons.wikimedia.org | More Like This | ||
| Keywords: Megalocystidium-Minimum Evolution-Tree.svg Figure Evolutionary relationship of Megalocystidium <br/> The evolutionary history was inferred using the Minimum Evolution method 1 The optimal tree with the sum of branch length 0 36807172 is shown The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test 1000 replicates are shown next to the branches 2 The tree is drawn to scale with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree The evolutionary distances were computed using the Kimura 2-parameter method 3 and are in the units of the number of base substitutions per site The ME tree was searched using the Close-Neighbor-Interchange CNI algorithm 4 at a search level of 2 The Neighbor-joining algorithm 5 was used to generate the initial tree The analysis involved 20 nucleotide sequences All positions with less than 95 site coverage were eliminated That is fewer than 5 alignment gaps missing data and ambiguous bases were allowed at any position Evolutionary analyses were conducted in http //megasoftware net/ MEGA6 2<br/> 1 Rzhetsky A and Nei M 1992 A simple method for estimating and testing minimum evolution trees Molecular Biology and Evolution 9 945-967 <br/> 2 Felsenstein J 1985 Confidence limits on phylogenies An approach using the bootstrap Evolution 39 783-791 <br/> 3 Kimura M 1980 A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences Journal of Molecular Evolution 16 111-120 <br/> 4 Nei M and Kumar S 2000 Molecular Evolution and Phylogenetics Oxford University Press New York <br/> 5 Saitou N and Nei M 1987 The neighbor-joining method A new method for reconstructing phylogenetic trees Molecular Biology and Evolution 4 406-425 <br/> 6 Tamura K Stecher G Peterson D Filipski A and Kumar S 2013 MEGA6 Molecular Evolutionary Genetics Analysis version 6 0 Molecular Biology and Evolution30 2725-2729 <br/> ; List of GenBank-Sequences http //www ncbi nlm nih gov/nuccore/AF506400 Acanthophysellum lividocoeruleum AF506400 http //www ncbi nlm nih gov/nuccore/AF506398 Aleurobotrys botryosus AF506398 http //www ncbi nlm nih gov/nuccore/AF506399 Aleurodiscus cerussatus AF506399 http //www ncbi nlm nih gov/nuccore/AF506382 Conferticium ravum AF506382 http //www ncbi nlm nih gov/nuccore/AF506432 Gloeocystidiellum aspellum AF506432 http //www ncbi nlm nih gov/nuccore/AF506434 Gloeocystidiellum compactum AF506434 http //www ncbi nlm nih gov/nuccore/AF506439 Gloeocystidiellum formosanum AF506439 http //www ncbi nlm nih gov/nuccore/AF506444 Gloeodontia columbiensis AF506444 http //www ncbi nlm nih gov/nuccore/AF506445 Gloeodontia discolor AF506445 http //www ncbi nlm nih gov/nuccore/AF506446 Gloeodontia pyramidata AF506446 http //www ncbi nlm nih gov/nuccore/AF506448 Gloeomyces graminicola AF506448 http //www ncbi nlm nih gov/nuccore/AF506441 Megalocystidium chelidonium AF506441 http //www ncbi nlm nih gov/nuccore/AF506420 Megalocystidium leucoxanthum AF506420 http //www ncbi nlm nih gov/nuccore/AF506421 Megalocystidium luridum AF506421 http //www ncbi nlm nih gov/nuccore/AF506422 Megalocystidium luridum AF506422 http //www ncbi nlm nih gov/nuccore/AF506443 Megalocystidium wakullum AF506443 http //www ncbi nlm nih gov/nuccore/AF506479 Stereum hirsutum AF506479 http //www ncbi nlm nih gov/nuccore/AF506480 Stereum reflexulum AF506480 http //www ncbi nlm nih gov/nuccore/AF506491 Xylobolus frustulatus AF506491 http //www ncbi nlm nih gov/nuccore/AF506397 Aleurodiscus amorphus AF506397 own 2014-09-09 Thkgk Cc-zero other versions Megalocystidium | ||||