MAKE A MEME
View Large Image
| View Original: | Image_from_page_182_of_"Practical_physics"_(1922).jpg (778x572) | |||
| Download: | Original | Medium | Small | Thumb |
| Courtesy of: | www.flickr.com | More Like This | ||
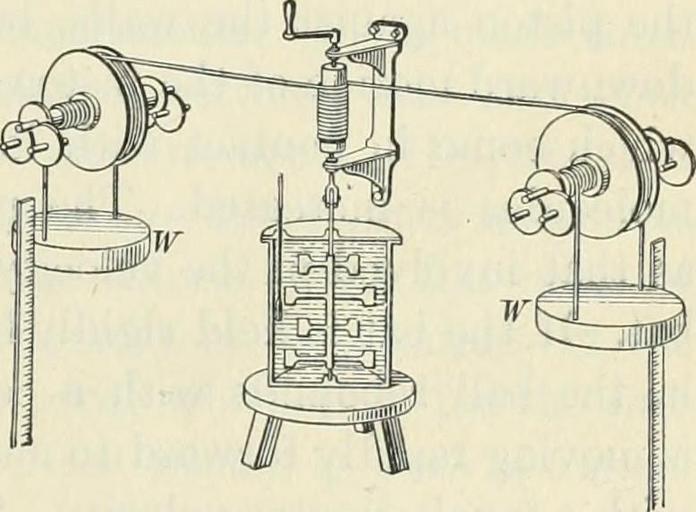
| Keywords: bookid:practicalphysics00mill bookidpracticalphysics00mill bookyear:1922 bookyear1922 bookdecade:1920 bookdecade1920 bookcentury:1900 bookcentury1900 bookauthor:millikan__robert_andrews bookauthormillikanrobertandrews bookauthor:gale__henry_gordon bookauthorgalehenrygordon booksubject:physics booksubjectphysics bookpublisher:boston___ginn_and_co_ bookpublisherbostonginnandco bookcontributor:university_of_british_columbia_library bookcontributoruniversityofbritishcolumbialibrary booksponsor:university_of_british_columbia_library booksponsoruniversityofbritishcolumbialibrary bookleafnumber:182 bookleafnumber182 bookcollection:ubclibrary bookcollectionubclibrary bookcollection:toronto bookcollectiontoronto sketch drawing text illustration cartoon writing monochrome bookid:practicalphysics00mill bookidpracticalphysics00mill bookyear:1922 bookyear1922 bookdecade:1920 bookdecade1920 bookcentury:1900 bookcentury1900 bookauthor:millikan__robert_andrews bookauthormillikanrobertandrews bookauthor:gale__henry_gordon bookauthorgalehenrygordon booksubject:physics booksubjectphysics bookpublisher:boston___ginn_and_co_ bookpublisherbostonginnandco bookcontributor:university_of_british_columbia_library bookcontributoruniversityofbritishcolumbialibrary booksponsor:university_of_british_columbia_library booksponsoruniversityofbritishcolumbialibrary bookleafnumber:182 bookleafnumber182 bookcollection:ubclibrary bookcollectionubclibrary bookcollection:toronto bookcollectiontoronto sketch drawing text illustration cartoon writing monochrome Identifier: practicalphysics00mill Title: Practical physics Year: 1922 (1920s) Authors: Millikan, Robert Andrews Gale, Henry Gordon Subjects: Physics Publisher: Boston : Ginn and Co. Contributing Library: University of British Columbia Library Digitizing Sponsor: University of British Columbia Library View Book Page: Book Viewer About This Book: Catalog Entry View All Images: All Images From Book Click here to view book online to see this illustration in context in a browseable online version of this book. Text Appearing Before Image: weights in causing them to descendthrough any distance (/ was equalto their weight W tinies this dis-tance. If the weights descendedslowly and uniformly, this workwas all expended in overcomingthe resistance of the water tothe motion of the paddle wheelsthrough it; that is, it was wastedin eddy currents in the water.Joule measured the rise in thetemperature of the water andfound that the mean of his threebest trials gave 427 gram metersas the amount of work required to d-evelop enough heat to raise a gram of water one degree. This value,confirmed by modern experiments, is now generally accepted as correct.He then repeated the experiment, substituting mercury for water, andobtained 12.5 gram meters as the work necessary to produce a calorie ofheat. The difference between these numbers is less than was to havebeen expected from the unavoidable errors in the observations. Hethen devised an arrangement in which the heat was developed by thefriction of iron on iron, and again obtained 425. Text Appearing After Image: Fig. 108. Joules first experiment onthe mechanical eqvuvalent of heat 154 WORK AJsD HEAT ENERGY 184. Heat produced by collision. A Frenchman namedHirn was the first to make a careful cletermmation of therelation between the heat developed by collision and the kineticenergy which disappears. lie allowed a steel cylinder to fallthrough a known height and crush a lead ball by its impactupon it. The amount of heat developed in the lead was meas-ured by observing the rise in temperature of a small amount ofwater into which the lead was quickly plunged. As the meanof a large number of trials he also found that 425 gram metersof energy disappeared for each calorie of heat that appeared. 185. Heat produced by the compression of a gas. Another wayin which Joule measured the relation between heat and workwas by compressing a gas and comparing the amount of workdone in the compression with the amount of heat developed. Every bicyclist is aware of the fact tliat when he inflates histires the pump Note About Images Please note that these images are extracted from scanned page images that may have been digitally enhanced for readability - coloration and appearance of these illustrations may not perfectly resemble the original work. Identifier: practicalphysics00mill Title: Practical physics Year: 1922 (1920s) Authors: Millikan, Robert Andrews Gale, Henry Gordon Subjects: Physics Publisher: Boston : Ginn and Co. Contributing Library: University of British Columbia Library Digitizing Sponsor: University of British Columbia Library View Book Page: Book Viewer About This Book: Catalog Entry View All Images: All Images From Book Click here to view book online to see this illustration in context in a browseable online version of this book. Text Appearing Before Image: weights in causing them to descendthrough any distance (/ was equalto their weight W tinies this dis-tance. If the weights descendedslowly and uniformly, this workwas all expended in overcomingthe resistance of the water tothe motion of the paddle wheelsthrough it; that is, it was wastedin eddy currents in the water.Joule measured the rise in thetemperature of the water andfound that the mean of his threebest trials gave 427 gram metersas the amount of work required to d-evelop enough heat to raise a gram of water one degree. This value,confirmed by modern experiments, is now generally accepted as correct.He then repeated the experiment, substituting mercury for water, andobtained 12.5 gram meters as the work necessary to produce a calorie ofheat. The difference between these numbers is less than was to havebeen expected from the unavoidable errors in the observations. Hethen devised an arrangement in which the heat was developed by thefriction of iron on iron, and again obtained 425. Text Appearing After Image: Fig. 108. Joules first experiment onthe mechanical eqvuvalent of heat 154 WORK AJsD HEAT ENERGY 184. Heat produced by collision. A Frenchman namedHirn was the first to make a careful cletermmation of therelation between the heat developed by collision and the kineticenergy which disappears. lie allowed a steel cylinder to fallthrough a known height and crush a lead ball by its impactupon it. The amount of heat developed in the lead was meas-ured by observing the rise in temperature of a small amount ofwater into which the lead was quickly plunged. As the meanof a large number of trials he also found that 425 gram metersof energy disappeared for each calorie of heat that appeared. 185. Heat produced by the compression of a gas. Another wayin which Joule measured the relation between heat and workwas by compressing a gas and comparing the amount of workdone in the compression with the amount of heat developed. Every bicyclist is aware of the fact tliat when he inflates histires the pump Note About Images Please note that these images are extracted from scanned page images that may have been digitally enhanced for readability - coloration and appearance of these illustrations may not perfectly resemble the original work. Identifier: practicalphysics00mill Title: Practical physics Year: 1922 (1920s) Authors: Millikan, Robert Andrews Gale, Henry Gordon Subjects: Physics Publisher: Boston : Ginn and Co. Contributing Library: University of British Columbia Library Digitizing Sponsor: University of British Columbia Library View Book Page: Book Viewer About This Book: Catalog Entry View All Images: All Images From Book Click here to view book online to see this illustration in context in a browseable online version of this book. Text Appearing Before Image: weights in causing them to descendthrough any distance (/ was equalto their weight W tinies this dis-tance. If the weights descendedslowly and uniformly, this workwas all expended in overcomingthe resistance of the water tothe motion of the paddle wheelsthrough it; that is, it was wastedin eddy currents in the water.Joule measured the rise in thetemperature of the water andfound that the mean of his threebest trials gave 427 gram metersas the amount of work required to d-evelop enough heat to raise a gram of water one degree. This value,confirmed by modern experiments, is now generally accepted as correct.He then repeated the experiment, substituting mercury for water, andobtained 12.5 gram meters as the work necessary to produce a calorie ofheat. The difference between these numbers is less than was to havebeen expected from the unavoidable errors in the observations. Hethen devised an arrangement in which the heat was developed by thefriction of iron on iron, and again obtained 425. Text Appearing After Image: Fig. 108. Joules first experiment onthe mechanical eqvuvalent of heat 154 WORK AJsD HEAT ENERGY 184. Heat produced by collision. A Frenchman namedHirn was the first to make a careful cletermmation of therelation between the heat developed by collision and the kineticenergy which disappears. lie allowed a steel cylinder to fallthrough a known height and crush a lead ball by its impactupon it. The amount of heat developed in the lead was meas-ured by observing the rise in temperature of a small amount ofwater into which the lead was quickly plunged. As the meanof a large number of trials he also found that 425 gram metersof energy disappeared for each calorie of heat that appeared. 185. Heat produced by the compression of a gas. Another wayin which Joule measured the relation between heat and workwas by compressing a gas and comparing the amount of workdone in the compression with the amount of heat developed. Every bicyclist is aware of the fact tliat when he inflates histires the pump Note About Images Please note that these images are extracted from scanned page images that may have been digitally enhanced for readability - coloration and appearance of these illustrations may not perfectly resemble the original work. Identifier: practicalphysics00mill Title: Practical physics Year: 1922 (1920s) Authors: Millikan, Robert Andrews Gale, Henry Gordon Subjects: Physics Publisher: Boston : Ginn and Co. Contributing Library: University of British Columbia Library Digitizing Sponsor: University of British Columbia Library View Book Page: Book Viewer About This Book: Catalog Entry View All Images: All Images From Book Click here to view book online to see this illustration in context in a browseable online version of this book. Text Appearing Before Image: weights in causing them to descendthrough any distance (/ was equalto their weight W tinies this dis-tance. If the weights descendedslowly and uniformly, this workwas all expended in overcomingthe resistance of the water tothe motion of the paddle wheelsthrough it; that is, it was wastedin eddy currents in the water.Joule measured the rise in thetemperature of the water andfound that the mean of his threebest trials gave 427 gram metersas the amount of work required to d-evelop enough heat to raise a gram of water one degree. This value,confirmed by modern experiments, is now generally accepted as correct.He then repeated the experiment, substituting mercury for water, andobtained 12.5 gram meters as the work necessary to produce a calorie ofheat. The difference between these numbers is less than was to havebeen expected from the unavoidable errors in the observations. Hethen devised an arrangement in which the heat was developed by thefriction of iron on iron, and again obtained 425. Text Appearing After Image: Fig. 108. Joules first experiment onthe mechanical eqvuvalent of heat 154 WORK AJsD HEAT ENERGY 184. Heat produced by collision. A Frenchman namedHirn was the first to make a careful cletermmation of therelation between the heat developed by collision and the kineticenergy which disappears. lie allowed a steel cylinder to fallthrough a known height and crush a lead ball by its impactupon it. The amount of heat developed in the lead was meas-ured by observing the rise in temperature of a small amount ofwater into which the lead was quickly plunged. As the meanof a large number of trials he also found that 425 gram metersof energy disappeared for each calorie of heat that appeared. 185. Heat produced by the compression of a gas. Another wayin which Joule measured the relation between heat and workwas by compressing a gas and comparing the amount of workdone in the compression with the amount of heat developed. Every bicyclist is aware of the fact tliat when he inflates histires the pump Note About Images Please note that these images are extracted from scanned page images that may have been digitally enhanced for readability - coloration and appearance of these illustrations may not perfectly resemble the original work. | ||||