MAKE A MEME
View Large Image
| View Original: | CCTS_CI_Use_Case.png (1078x910) | |||
| Download: | Original | Medium | Small | Thumb |
| Courtesy of: | commons.wikimedia.org | More Like This | ||
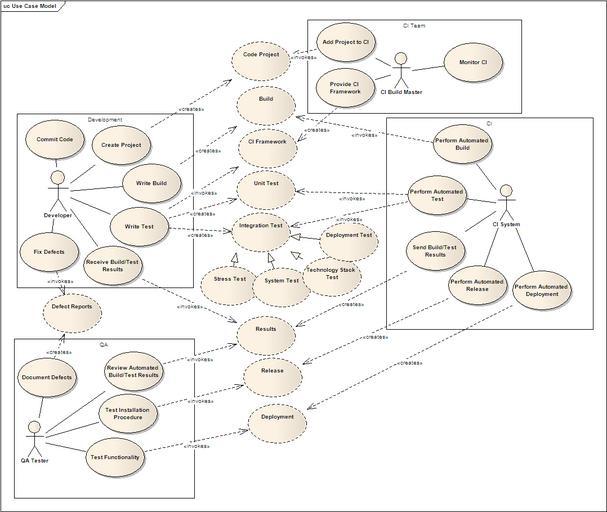
| Keywords: CCTS CI Use Case.png en Use Case View on CCTS Continuous Integration Architecture The caBIG Clinical Trials Suite CCTS is an enterprise clinical trials system being designed primarily for use in trial sites The suite is comprised of a collection of interoperable modules covering a broad range of key areas in cancer clinical trials management These include patient registration via C3PR patient scheduling via PSC adverse events reporting via caAERS lab analysis via LabViewer and clinical data management via C3D Integration between these applications is centered around five key scenarios Study Creation Register Subject Load Labs in CDMS Lab-driven AE Creation and AE-Triggered Schedule Change The implementation is based upon the caGrid infrastructure with caXchange as the Enterprise Service Bus for reliable message routing and GAARDS providing robust security https //cabig-kc nci nih gov/CTMS/KC/index php/Image CCTS_CI_Use_Case png PMC Connell at nih gov 2008-09-23 PD-USGov Use Case diagrams | ||||