MAKE A MEME
View Large Image
| View Original: | Bohr-model-Helium.gif (252x247) | |||
| Download: | Original | Medium | Small | Thumb |
| Courtesy of: | commons.wikimedia.org | More Like This | ||
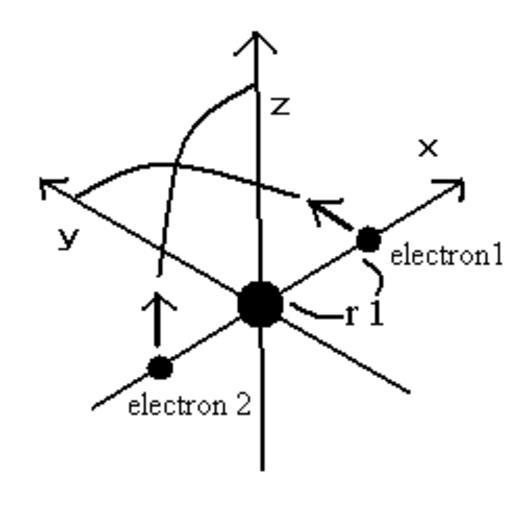
| Keywords: Bohr-model-Helium.gif en Bohr model of Helium __ This model is just consistent with the experimental value of helium ground state energy Two same-shaped orbital not circular planes of electrons are perpendicular to each other This figure shows one quarter of the orbitals Electron 1 starts at r1 0 0 while electron 2 starts at -r1 0 0 By a computational method we calculate the Coulomb force among the particles and the number of de Broglie's waves contained in the short segment at short time intervals The orbital length is proved to be just one times de Broglie's wavelength See http //arxiv org/abs/0903 2546 in detail Own Ptrygne 2009-03-28 Bohr model Helium | ||||